
Introduction
India is the 4th largest producer of ethanol after United States of America (USA), China, and Brazil having more than 300 distilleries with a production capacity of about 3.2 billion liters mainly by fermentation of sugarcane molasses. Out of the total available ethanol, about 45% is used for potable liquor, about 40% is used as industrial solvent in chemical industry, for blending in fuel and for other applications. There is a continuous growth in demand for ethanol in India due to growth in industrial applications and its use as blending agent in fuel. However, the production capacities are lagging with respect to the market demand for the ethanol and the amount of ethanol currently being produced in India is not sufficient to meet domestic demand. The ongoing efforts to reduce the fuel import burden on the country, the Central government’s decision to increase the blending percentage of alcohol in petrol is encouraging the ethanol producers to increase their capacities and to explore alternate feedstock for ethanol fermentation. This has enabled sugar mills to produce ethanol by diverting cane juice/Syrup towards ethanol production and availability of ethanol for the Ethanol Blended Petrol (EBP) program. The support from government to provide higher compensation price for ethanol produced from sugar juice or syrup and B molasses would aid in reducing excess sugar stocks and impacting the sugar industry capacity to pay arrears to sugarcane farmers. All sugar mills/ distilleries are planning to take benefit of the scheme by participating in supply ethanol for the Ethanol Blended Petrol (EBP) program.
India and Brazil are the largest producers of sugarcane in the world primarily to produce sugar. Molasses is the key by product of sugar industry and is one of the main feedstocks for ethanol production, however in future sugarcane juice and syrup will also have a significant share as a feedstock for ethanol fermentation that may include primary/secondary/mixed/clear juice and syrup which is concentrated juice with total dissolved solid content more than 50 Brix. Clarified sugarcane juice, from the milling section, is concentrated to 60 brix in the falling film evaporation section to convert juice into syrup (Clark, 2013).
Operating the distillery throughout the year is a challenge without the storage of raw material for off-season, though cane juice can be used during season, it is very difficult to maintain its sugar content as well as keep it free from microbial contamination. However, the cane syrup could be stored and utilized as a raw material for off-season consumption. Owing to the rising demand for renewable bioenergy, there is a need for a sustainable solution to preserve sugarcane syrup for ethanol production. India, being a country committed to use of renewable energy under EBP20 program, has brought out an extensive plan for encouraging the production of bioethanol. Therefore, distillery industry in India has ample amount of growth opportunities to contribute in EBP program by preserving the excess sugarcane Syrup during crushing season and use the same to produce ethanol during off-season.
Challenges in syrup preservations:
The solubility of sucrose changes with varying temperature and at low temperatures the sucrose might crystallize in syrup (Serna-Saldivaret al., 2008). With the changing environmental conditions, the sucrose in syrup tends to breakdown into monomers, which are more susceptible for microbial degradation (Thompson, 2009). The neutral/ near neutral pH of the syrup is also a favorable condition for the microbial flora that enhances the microbial growth and syrup degradation (Vanderzant, 1992). Providing the right environment is necessary for syrup stability by maintaining pH and temperature conditions (Kapuret al.1978, Kunitakeet al., 2014). The pH is an indicator for microbial degradation and is also evident from formation of by-products such as Gluconic acid, Lactic acid, and Acetic acid (Madan et al., 1997, Rawat, 2015). The syrup stored without taking proper precautions may also decrease the fermentability of the syrup and hamper the ethanol production process.
Numerous preservation methods being investigated by researchers for the sugarcane syrup consists of chemical, thermal, and non-thermal methods that includes usage of enzymes prior to storage (Anejaet al., 2014, Kaavya, et al., 2019, Killerbyet al., 2022). Enzymes are also dosed in the storage tank at regular intervals for achieving extended shelf life of cane syrup. Simultaneously, the stored syrup will be cooled by recirculation with a provision of inert gas blanketing in the storage tank head space to avoid contamination when the syrup comes in to contact with air.
Cane Syrup Preservation Lab scale studies:
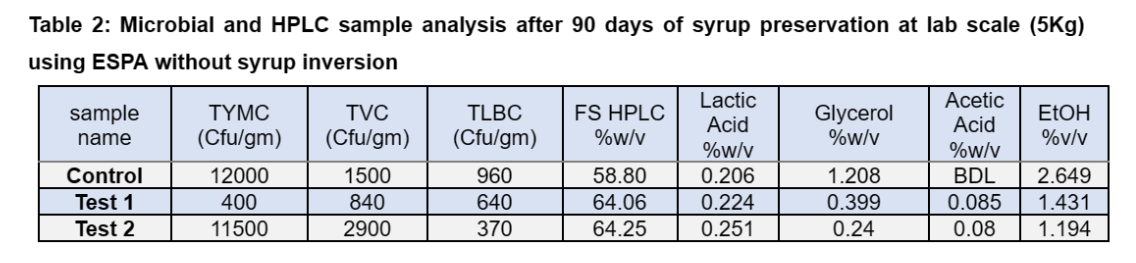
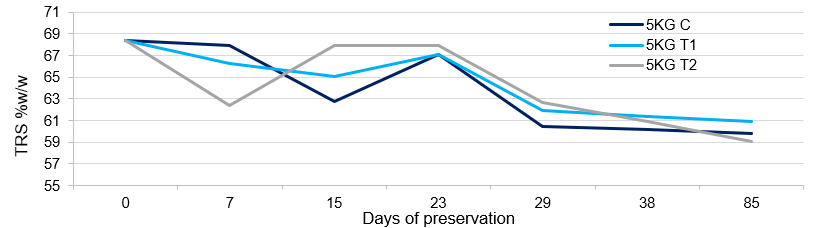
The lab scale studies were conducted at Catalysts Biotechnologies Research and Development center by sourcing the syrup from sugar industry having Brix of 76.04% and TRS 68.35%. The syrup was treated with Enzysyrup Protect Advance (ESPA), a proprietary blend of antimicrobial enzymes that prevents the osmotolerant bacteria and other microbial flora, at 20 ppm of dosage without inversion and preserved for 90days at lab scale (5Kg). During this tenure, considerable reduction in TRS, Brix with crystalized sugar at the bottom of the flask was observed (Table 1).
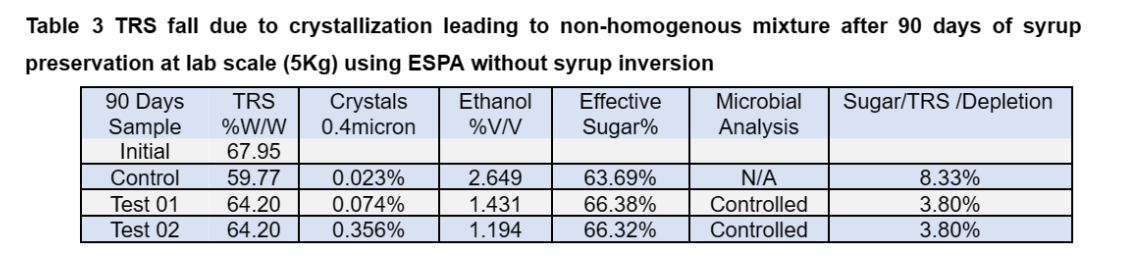
The sedimentation of crystals leads to non-homogeneity of the syrup and challenges during the fermentation. The microbial analysis of the preserved samples showed control of the microbial growth up to some extent (Table 2).
This clearly indicates that addition of preservative alone is not sufficient to preserve the cane syrup with good fermentability. The observations indicate total sugar of the treated syrup (TRS%+Crystalize sugar %) was stable during the trial period whereas the total sugar of control samples was reduced by 8%. The data is tabulated in Table 3 and the slight variations of the analysis can be attributed to the evaporation and fine crystallization remain un-sedimented.
The TRS trend of syrup preservation without inversion is depicted in Figure 1.
Catalysts Combinatorial Approach to preserve Cane Syrup:
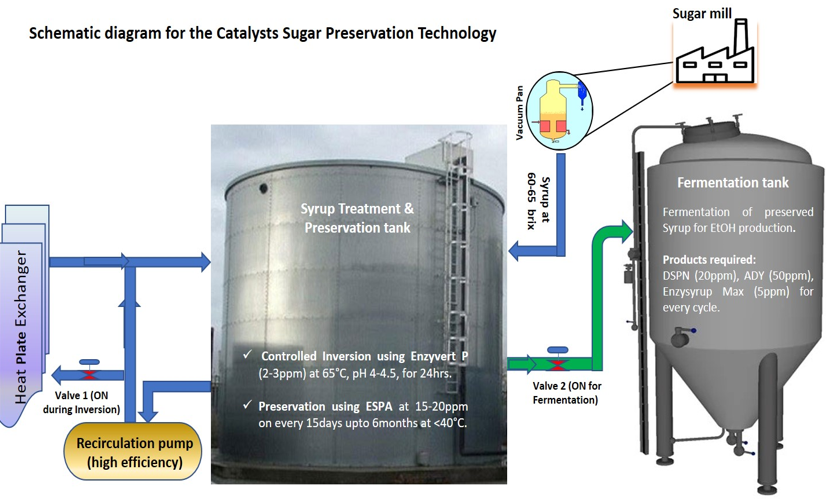
Based on the lab scale results Catalysts has developed a unique, innovative patented technology for syrup storage with the help of proprietary enzyme formulations using combinatorial approach that provide cost effective tailor-made solution to the industry. The syrup preservation approach developed by Catalysts Biotechnologies includes enzymatic treatment of sugar syrup followed by antimicrobial treatment during storage. The preservation process starts by diverting the syrup of > 65 Brix with TRS > 58%w/w from the vacuum evaporation pan to a storage vessel. During the diversion process the syrup is treated to have the right environment for the enzyme formulation (EnzyInvert P Conc., 2-3 ppm) to work via providing the necessary co-factors, desired pH and temperature conditions The storage vessel can be equipped with temperature control system using via recirculation pump and a dosing pump. The controlled inversion process makes the syrup stable and can be preserved at ambient temperatures without degradation and crystallization for up to 6-9 months. To enhance the shelf life of the syrup, ESPA will be dosed at 15-20 ppm weekly/fortnightly based on the environmental conditions of the plant. The broad overview of the preservation process is depicted in Figure 2.
Figure 2: Schematic Diagram for the Treatment of Syrup using Catalysts’ Combinatorial Technology
Pilot Scale (35KL) Study of Catalysts Syrup Preservation Technology
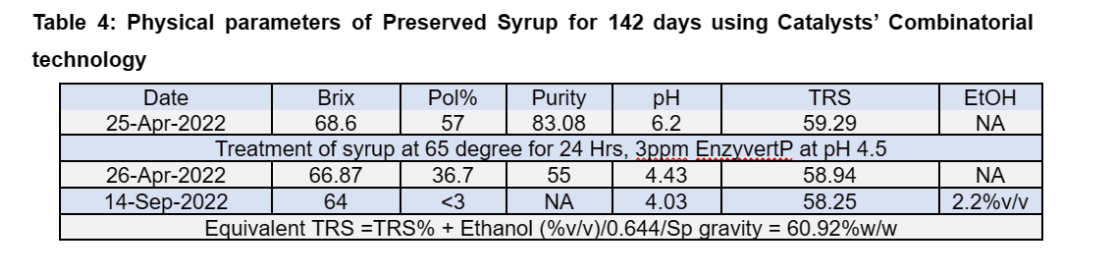
The pilot-scale preservation study was conducted with 35,000 L syrup having 64% Brix and TRS of 59.29%. The treatment was completed in 24 hours and the syrup was stored for 142 days with fortnightly addition of preservative enzymes.
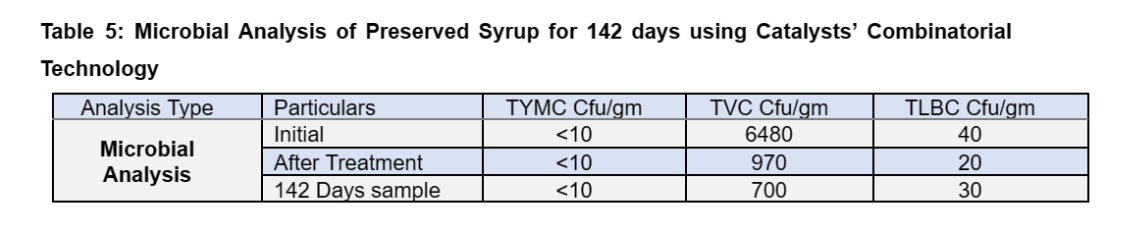
The observations of the trial are depicted in Table 4, which shows stable values of the TRS and Brix and a one log reduction in microbial load compared to initial microbial count (Table 5).
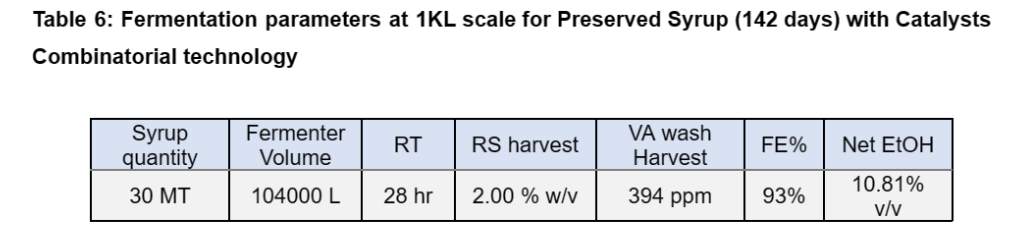
After 142 days of storage the syrup was checked for fermentability. The fermentation study was conducted with Catalysts ADY (50ppm), DSPN Pro (20ppm) and Enzysyrup max (5ppm). The fermentation study results are shown in Table 6 with a good fermentation efficiency (FE) of 92-93%.
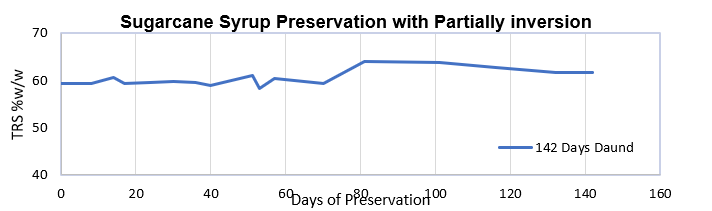
The TRS trend of syrup sample is depicted in Figure 3.
Figure 3: TRS trend of preserved syrup for 142 days, treated using combinatorial Technology
Discussion
To support the ethanol blending program by Govt. of India, several sugar industries are coming up with distillery division to have flexibility of sugar and ethanol production as per market demand. In addition to this, the ethanol produced from sugarcane is considered as a green fuel and there is a huge market demand for the same. To meet the market demand and to support sustainability availability of syrup throughout the year is a major challenge. The research community is constantly working on addressing these challenges. This unique combinatorial approach developed by Catalysts Biotechnologies Pvt. Ltd. is going to be a game changer in this segment for prolonged storage of sugarcane syrup that enables sugar industries to store syrup during off season up to 6-9 months to continuously.
Advantages of Catalysts Syrup Preservation technology:
1. The process is cost effective and requires minimal infrastructure.2. Controlled inversion process addresses the crystallization during storage.
3. Reduced microbial activity due to the antimicrobial enzyme ESPA.
4. The ease of handling syrup during storage and fermentation due to reduction in viscosity.
5. Enhanced fermentability with reduced retention time at high gravity fermentation 6. Depolymerization of complex sugars like Dextran & starch in the syrup that supports yeast metabolism for achieving better FE.
Acknowledgement
We thank our Managing director for his encouragement and providing Infrastructure. Our special thanks to Wave Industries, Dhanaura, Daund Sugar Factory, Daund, for providing raw materials. We also thank Aniket, Joole, Prerna, Kuldeep, Hari Mate, Sandeep, and Sathya Sundar for their support during experiments.
References
1. Aneja, K.R., Dhiman, R., Aggarwal, N.K. and Aneja, A., 2014. Emerging preservation techniques for controlling spoilage and pathogenic microorganisms in fruit juices. International journal of microbiology, 2014.2. Clarke, M., 2013, October. Chemistry and Processing of Sugarbeet and Sugarcane, edited by MA Clarke and MA Godshall. In Chemistry and Processing of Sugarbeet and Sugarcane: Proceedings of the Symposium on the Chemistry and Processing of Sugarbeet, Denver, Colorado, April 6, 1987 and the Symposium on the Chemistry and Processing of Sugarcane, New Orleans, Louisiana, September 3-4, 1987 (p. 162). Elsevier.
3. Kaavya, R., Pandiselvam, R., Kothakota, A., Banuu Priya, E.P. and Arun Prasath, V., 2019. Sugarcane juice preservation: A critical review of the state of the art and way forward. Sugar Tech, 21(1), pp.9-19.
4. Kapur, K.L., Singh, V.P., Garg, R.G. and Agarwal, M.P., 1978. Preliminary studies on preservation of sugarcane juice. Indian food packer.
5. Killerby, M.A., Reyes, D.C., White, R. and Romero, J.J., 2022. Meta-analysis of the effects of chemical and microbial preservatives on hay spoilage during storage. Journal of Animal Science, 100(3), p.skac023.
6. Kunitake, M., Ditchfield, C., Silva, C. and Petrus, R., 2014. Effect of pasteurization temperature on stability of an acidified sugarcane juice beverage. Ciência e Agrotecnologia, 38(6), pp.554-561.
7. Madan, V.K., Misra, S.R., Soni, N. and Solomon, S., 1997. Manual for sugar analysis. IISR, Lucknow, India.
8. Rawat, S., 2015. Food Spoilage: Microorganisms and their prevention. Asian journal of plant science and Research, 5(4), pp.47-56.
9. Serna-Saldivar, S.R. and Rito-Palomares, M.A., Instituto Technologico y de Estudios Superiores de Monterrey, 2008. Production of invert syrup from sugarcane juice using immobilized invertase. U.S. Patent 7,435,564.
10. Thompson, S., 2009. Microbiological spoilage of high-sugar products. In Compendium of the microbiological spoilage of foods and beverages (pp. 301-324). Springer, New York, NY.
11. Vanderzant, C., 1992. Compendium of methods for the microbiological examination of foods. Indicator Microorganisms and Pathogens, pp.337-338.
Recent Posts

Rapid Corn Oil Estimation with Near-Infrared Spectroscopy: An Effective Analytical Technique with Ne
Explore the effectiveness of near-infrared spectroscopy in rapidly estimating corn oil, providing new perspectives and enhancing analytical techniques in agriculture.
GC-FID: A tool for analysis of FAMEs in corn oil
Explore GC-FID, the essential tool for accurate FAME analysis in corn oil. Unlock detailed insights and improve your research outcomes today.

The Role of Enzymes in Maize-to-Ethanol Fermentation
With the rising demand for renewable energy sources and sustainable fuel alternatives, ethanol production has gained significant momentum worldwide. Ethanol, also known as bioethanol, is a biofuel commonly used as a renewable alternative to fossil fuels. One of the most popular feedstocks for ethanol production is maize (corn), primarily due to its high starch content, wide availability, and suitability for large-scale production.
Catalysts Connect
Keep up to date with our latest news and analysis by subscribing to our regular magazine and newsletter

1 Comment
Amber
+574 days agoHello thecatalystsgroup.com admin, Your posts are always well-supported by facts and figures.
Post a comment
Your email address will not be published.